C1 Metabolism in Trees
The Science
An oxidative C1 pathway is known to exist in plants where intermediates with a single carbon atom beginning with methanol are oxidized to CO2. Although the flux of carbon through the C1 pathway is thought to be large, its intermediates are difficult to measure and relatively little is known about this potentially ubiquitous and mysterious pathway. In this study, we evaluated the C1 pathway and its integration with central metabolism using aqueous solutions of 13C-labeled C1 and C2 intermediates delivered to branches of the tropical species Inga edulis via the transpiration stream.
The Impact
Our results demonstrate that methanol activates the C1 pathway in plants which provides an alternative carbon source for glycine methylation in photorespiration, enhance CO2 concentrations within chloroplasts, and produce key C2 intermediates (e.g. acetyl-CoA) for central metabolism. Our observations are consistent with previous studies that demonstrated formaldehyde integrates into photorespiration in the mitochondria by providing an alternate source of CH2-THF used for the methylation of serine to glycine. By eliminating the need for a second glycine for the production of CH2-THF with the subsequent loss of CO2 and NH3, the integration of C1 pathway into photorespiration may convert it from a net loss of carbon to a net gain. By also suppressing photorespiration via the production of CO2 in chloroplasts, our study presents the hypothesis that the integration of C1 pathway into C2/3 metabolism may boost carbon use efficiency and therefore represent an important mechanism by trees under photorespiratory conditions (e.g. high temperature stress). As agricultural crops are known to be high methanol producers, genetic manipulation of the C1 pathway has the potential to improve yields and tolerance to environmental extremes, thereby providing a new tool to the agriculture, bioenergy, and biomanufacturing industries.
Summary
Methanol is highly abundant in the global atmosphere and is known to be tightly connected to plant growth. However, to date, it is assumed that methanol represents a byproduct of the expansion of cell walls during growth processes. Although evidence for the existence of a C1 pathway in plants was first collected over 50 years ago, its intermediates are difficult to measure and relatively little is known about this potentially ubiquitous, yet mysterious biochemical pathway. Previous research by one of the founding fathers of photosynthesis research (Dr. Andrew Benson), for whom this paper is dedicated, found evidence for an important role of methanol in boosting plant photosynthesis, biomass, and productivity. However, this topic remains controversial as subsequent researchers were unable to observe these effects, and the biochemical mechanism(s) remain unclear.
In this paper, we employ the newly developed technique in our lab termed dynamic 13C-pulse chase to evaluate the potential existence of the complete C1 pathway and its integration with C2/3 metabolism in individual branches of a tropical pioneer species using aqueous solutions of 13C-labeled C1 (methanol, formaldehyde, formic acid) and C2 (acetic acid, glycine) intermediates delivered via the transpiration stream. We confirm that methanol initiates the complete C1 pathway in plants (methanol, formaldehyde, formic acid, carbon dioxide) by providing the first real-time dynamic 13C-labeling data showing their interdependence. We present novel aspects about the pathway including the rapid interconversion between methanol and formaldehyde, whereas once oxidation to formate occurs, it is quickly oxidized to CO2 within chloroplasts where it can be re-assimilated by photosynthesis. We show for the first time that reassimilation of C1, respiratory, and photorespiratory CO2 is a common mechanism for isoprene biosynthesis; a strong linear dependence of 13C-labeling of isoprene on 13C-labeling of CO2 was observed across all C1 and C2 13C-labeled substrates. Thus, this analysis presents a new method for studying the reassimilation of internal CO2 sources in plants. Finally, we show, for the first time, that methanol and formaldehyde delivery to the transpiration stream leads to a rapid and quantitative conversion of carbon pools used in the biosynthesis of central C2 compounds (acetic acid and acetyl CoA) and therefore represents a new uncharacterized route to the biosynthesis of these key C2 intermediates widely used in cells as precursors for a diverse suite of anabolic (e.g. fatty acid biosynthesis) and catabolic (e.g. mitochondrial respiration) processes.
Our observations are consistent with previous studies that demonstrated formaldehyde integrates into photorespiration in the mitochondrial by providing an alternate source of CH2-THF used for the methylation of serine to glycine. By eliminating the need for a second glycine for the production of CH2-THF with the subsequent loss of CO2 and NH3, the integration of C1 pathway into photorespiration may convert it from a net loss of carbon to a net gain. By also suppressing photorespiration via the production of CO2 in chloroplasts, our study presents the hypothesis that the integration of C1 pathway into C2/3 metabolism may boost carbon use efficiency during photorespiratory conditions (e.g. high temperature stress). As all agricultural crops have been shown to be high methanol producers, genetic manipulation of the C1 pathway has the potential to improve yields and tolerance to environmental extremes, thereby providing a new tool to the agriculture, bioenergy, and biomanufacturing industries.
Contacts (BER PM): Daniel Stover, SC-23.1, Daniel.Stover@science.doe.gov
PI Contact: Kolby J. Jardine, Lawrence Berkeley National Laboratory (LBNL), Climate and Ecosystem Sciences Division, kjjardine@lbl.gov
Funding
This material is based upon work supported as part of the GoAmazon 2014/5 and the Next Generation Ecosystem Experiments-Tropics (NGEE-Tropics) funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research through contract No. DE-AC02-05CH11231 to LBNL, as part of DOE’s Terrestrial Ecosystem Science Program. Additional funding for this research was provided by the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Publications
Jardine, K. et al. (2017), Integration of C1 and C2 Metabolism in Trees. International Journal of Molecular Sciences, 18, 2045, DOI:10.3390/ijms18102045
Related Links
http://www.mdpi.com/1422-0067/18/10/2045
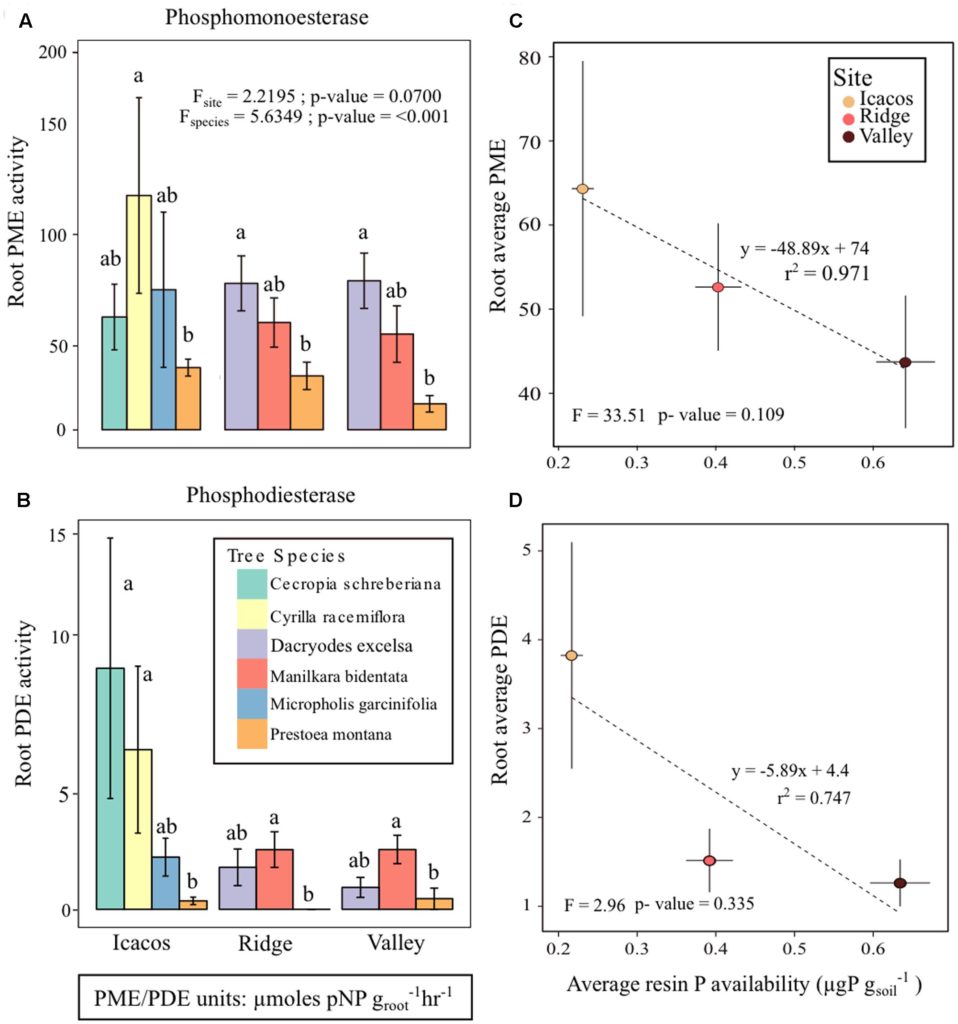
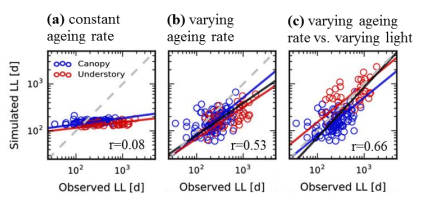 We use a trait-based carbon optimality approach to model leaf longevity (LL, in days), and assess the model performance with in-situ LL data for 105 species in two tropical forests in Panama. More specifically, we examine the relative impact of leaf ageing rate (i.e. the rate at which leaf photosynthetic capacity declines with age) and within-canopy variation in light environment on the modeled LL. We first assumed that all species have the same leaf ageing rate (i.e. the community average value) and receive the same light condition (i.e. canopy-level light), and the results are shown in panel a, with a correlation coefficient r=0.08 which is not significant. Then we performed the analysis with species-specific leaf ageing rates, while assuming that all species receive the same light condition (i.e. canopy-level light), and the results are shown in panel b, with r=0.53 and p-value<<0.001. We lastly performed the analysis with species-specific leaf ageing rate and light environment, and the results are shown in panel c, with r=0.66 and p-value <<0.001. Our results thus suggest that both leaf aging rate and within-canopy variation in light environment are essential for modeling LL in the tropics, and the best model can capture over 40% of interspecific variability in LL, including those species from canopy and understory.
We use a trait-based carbon optimality approach to model leaf longevity (LL, in days), and assess the model performance with in-situ LL data for 105 species in two tropical forests in Panama. More specifically, we examine the relative impact of leaf ageing rate (i.e. the rate at which leaf photosynthetic capacity declines with age) and within-canopy variation in light environment on the modeled LL. We first assumed that all species have the same leaf ageing rate (i.e. the community average value) and receive the same light condition (i.e. canopy-level light), and the results are shown in panel a, with a correlation coefficient r=0.08 which is not significant. Then we performed the analysis with species-specific leaf ageing rates, while assuming that all species receive the same light condition (i.e. canopy-level light), and the results are shown in panel b, with r=0.53 and p-value<<0.001. We lastly performed the analysis with species-specific leaf ageing rate and light environment, and the results are shown in panel c, with r=0.66 and p-value <<0.001. Our results thus suggest that both leaf aging rate and within-canopy variation in light environment are essential for modeling LL in the tropics, and the best model can capture over 40% of interspecific variability in LL, including those species from canopy and understory.